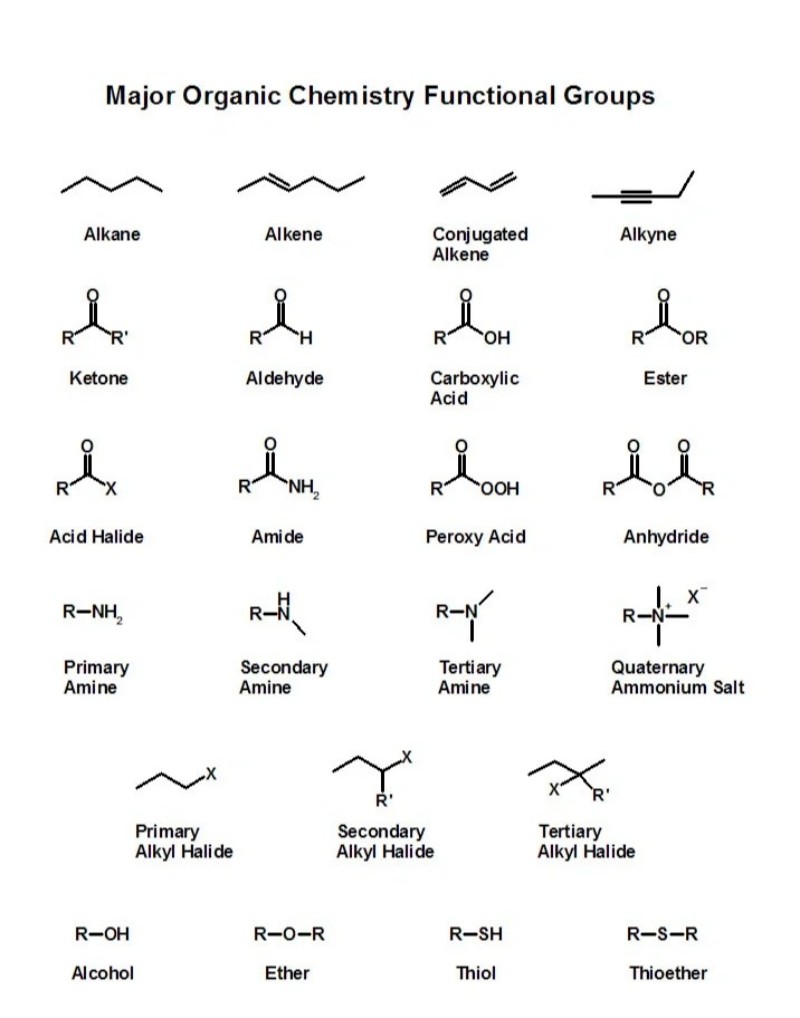
Katika kemia, hasa katika kemia ya kikaboni, kundi la utendaji kazi ni kundi maalum la atomi ndani ya molekuli ambalo huwajibika kwa athari za kemikali za molekuli. Lifikirie kama "eneo linalofanya kazi" au "sehemu inayofafanua tabia" ya molekuli ya kikaboni. Haijalishi ukubwa au umbo la molekuli iliyobaki ni nini, kundi la utendaji kazi hufanya kazi kwa njia inayoweza kutabirika katika athari za kemikali.
Kwa Nini Vikundi Vinavyofanya Kazi Ni Muhimu?
Makundi ya utendaji kazi huamua sifa na utendakazi wa misombo ya kikaboni. Wanakemia huzitumia kuainisha molekuli za kikaboni na kutabiri jinsi zitakavyoitikia. Kwa mfano, alkoholi, asidi, esta, na ketoni zote zina makundi tofauti ya utendaji kazi, na kila moja huguswa tofauti katika athari za kemikali.
Kwa kutambua makundi ya utendaji kazi katika molekuli, unaweza:
● Bashiri jinsi itakavyokuwa katika athari za kemikali.
● Elewa umumunyifu wake (iwe huyeyuka katika maji au la).
● Amua kama ni asidi au ya msingi.
● Bashiri kiwango chake cha kuchemka au kuyeyuka.
Mifano ya Vikundi vya Kazi vya Kawaida
Hebu tuangalie baadhi ya makundi ya utendaji kazi ya kawaida katika kemia ya kikaboni:
1. Kundi la Hidroksili (-OH)
● Inapatikana katika pombe.
● Hufanya molekuli kuwa za polar na zenye uwezo wa kutengeneza vifungo vya hidrojeni.
● Mfano: Ethanoli (CH₃CH₂OH)
2. Kundi la Kabonili (C=O)
● Inapatikana katika ketoni na aldehidi.
● Kaboni iliyounganishwa mara mbili na atomi ya oksijeni.
● Mfano:
Ketoni: Asetoni (CH₃COCH₃)
Aldehidi: Formaldehyde (HCHO)
3. Kundi la Kaboksili (-COOH)
● Inapatikana katika asidi ya kaboksiliki.
● Hufanya molekuli kuwa na asidi.
● Mfano: Asidi asetiki (CH₃COOH), asidi kuu katika siki.
4. Kundi la Amino (-NH₂)
● Inapatikana katika amini na amino asidi.
● Inaweza kutenda kama msingi na kukubali protoni.
● Mfano: Glycine, amino asidi.
5. Kundi la Ester (-COO-)
● Inapatikana katika esta.
● Mara nyingi hutoa matunda harufu zao tamu.
● Imetengenezwa kutokana na asidi na alkoholi.
● Mfano: Ethyl acetate (inayotumika katika kiondoa rangi ya kucha).
6. Kundi la Ether (ROR)
● Atomu ya oksijeni iliyounganishwa na makundi mawili ya kaboni.
● Kawaida katika viyeyusho.
● Mfano: Etha ya Diethili.
7. Kundi la Halide (CX)
● Ambapo X = halojeni kama F, Cl, Br, au I.
● Inapatikana katika halidi za alkyl.
● Hutumika katika vihifadhi joto na vizuia moto.
8. Kundi la Sulfhydryl (-SH)
● Inapatikana katika thiols.
● Sawa na hidroksili lakini pamoja na salfa.
● Muhimu katika muundo wa protini (vifungo vya disulfidi).
Vikundi vya Utendaji na Utendaji Mwitikio
Uwepo wa kikundi maalum cha utendaji kazi katika molekuli kwa kiasi kikubwa huamua jinsi molekuli hiyo itakavyoitikia. Kwa mfano:
● Alkoholi (-OH) zinaweza kukaushwa ili kuunda alkeni.
● Asidi za kaboksili (-COOH) zinaweza kuitikia na alkoholi na kuunda esta.
● Amini (-NH₂) zinaweza kutenda kama besi na kukubali ioni za hidrojeni.
Tabia hii inayoweza kutabirika ni muhimu sana katika kemia ya sintetiki, muundo wa dawa, na sayansi ya nyenzo.
Vikundi vya Kazi katika Molekuli za Kibiolojia
Makundi ya utendaji kazi pia ni muhimu kwa maisha. Katika biokemia, muundo na utendaji kazi wa protini, DNA, wanga, na mafuta hutegemea sana makundi ya utendaji kazi yaliyomo.
● Protini zina vikundi vya amino (-NH₂) na kaboksili (-COOH).
● Wanga mara nyingi huwa na vikundi vya hidroksili (-OH) na kabonili (C=O).
● DNA na RNA zina vikundi vya fosfeti (-PO₄) na besi zenye nitrojeni.
Makundi haya huruhusu molekuli za kibiolojia kuingiliana, kuunda vifungo vya hidrojeni, kuhamisha nishati, na zaidi.
Jinsi Wanakemia Wanavyotumia Vikundi Vinavyofanya Kazi
Wanakemia mara nyingi hutumia nukuu za kikundi zinazofanya kazi wakati wa kuchora au kutaja molekuli. Katika athari za kemikali, wanaweza kurejelea athari za kundi linalohusika. Kwa mfano:
● "Oxidation ya pombe" inarejelea athari zinazohusisha vikundi vya -OH.
● "Ubadilishaji wa nyuklia" mara nyingi huhusisha vikundi vya utendaji kazi wa halidi.
Pia hutumia uchanganuzi wa kikundi kinachofanya kazi ili kutambua misombo isiyojulikana kwa kutumia mbinu kama vile spektroskopia ya infrared (IR) na mwangwi wa sumaku ya nyuklia (NMR), kwa kuwa kila kundi hunyonya nishati kwa njia ya kipekee.
Muhtasari
Kundi la utendaji kazi ni kundi maalum la atomi katika molekuli linaloipa molekuli sifa na mwitikio wake maalum. Ni msingi wa kemia ya kikaboni, na kutoa njia ya kuainisha na kutabiri tabia ya molekuli changamano. Kuanzia alkoholi rahisi hadi DNA changamano, vikundi vya utendaji kazi husaidia kufafanua muundo, utendaji kazi, na mwitikio wa misombo ya kemikali. Kuzielewa ni muhimu kwa kuimudu kemia, hasa katika maeneo kama vile dawa, biolojia, na kemia ya viwanda.
Muda wa chapisho: Juni-20-2025